Pharmaceutical companies face unique hurdles: strict regulations, complex scientific messaging, reaching niche patient populations for clinical trials, and demonstrating value to diverse stakeholders like HCPs and payers. Standard marketing approaches often fall short.
We combine deep scientific understanding with cutting-edge marketing strategies. As scientists turned marketers, we translate complex information into compelling campaigns that resonate with your target audience, ensure compliance, and deliver measurable results, especially in critical areas like clinical trial recruitment.



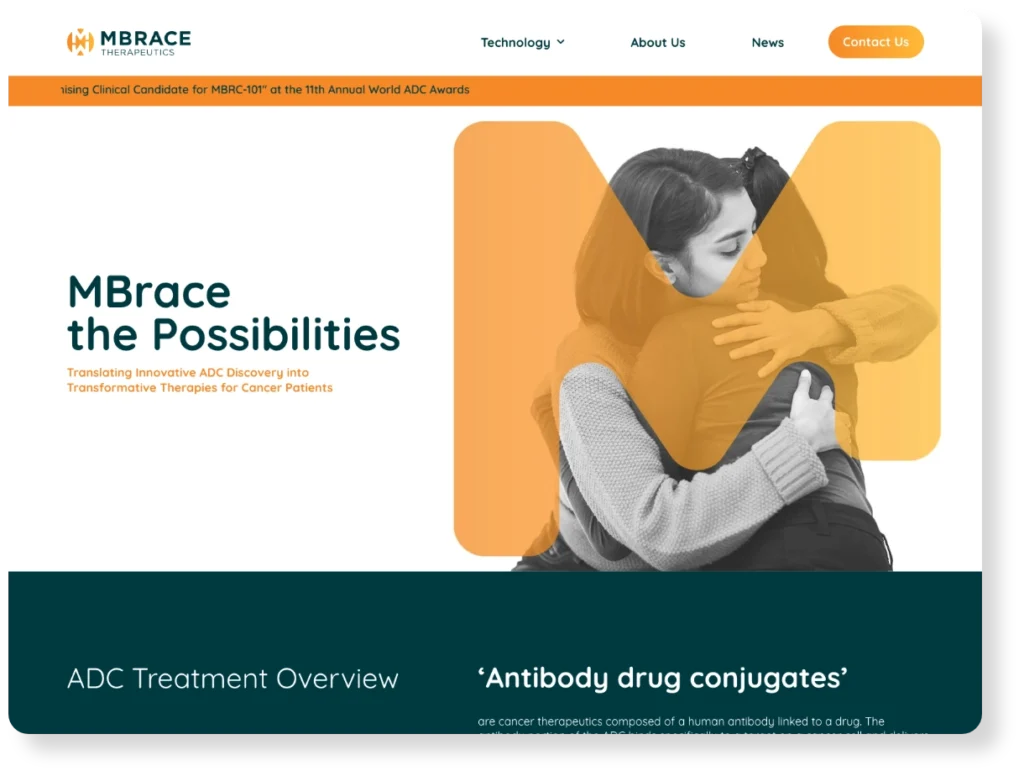
Targeted digital campaigns, patient journey mapping, and community outreach to identify, engage, and enroll qualified participants efficiently.
Data-driven strategies to reach and educate physicians and specialists through targeted advertising, webinars, and valuable content.
Expert management of Google Ads, LinkedIn Ads, and other platforms to maximize ROI and reach specific pharma audiences.
Developing clear, compliant, and compelling content (white papers, case studies, patient materials, videos) that communicates complex science effectively.
Enhancing online visibility for your therapies, trials, and brand through specialized SEO strategies for the life sciences sector.
In-depth market analysis, competitive intelligence, and strategic planning to guide successful product launches and campaigns.
We help pharma teams communicate complex data, reach clinical and scientific audiences, and support commercialization through targeted content and campaigns. Our approach blends scientific accuracy with clear messaging that resonates across R&D, clinical, and commercial functions.
We produce slide decks, one-pagers, MOA graphics, technical content, launch materials, and educational assets for scientific and clinical audiences. Every piece is developed with precision to meet the expectations of healthcare professionals and industry stakeholders.
Content is created and reviewed by PhD-level scientists and subject matter experts to ensure accuracy, clarity, and alignment with regulatory expectations. We work with your medical, regulatory, and commercial teams to keep messaging compliant and credible.
Pharma requires a higher level of rigor, careful claims handling, and clear communication tailored to clinicians, researchers, and regulatory bodies. The Samba team understands these nuances and builds materials that support both internal and external communication needs.
Timelines vary by complexity, but most materials take 2–4 weeks, including scientific review and compliance steps. Larger multi-asset projects or launch packages may require 4–8 weeks to allow for stakeholder approvals.