In 1918, the Spanish Flu infected 500 million people–that’s 1/3 of the global population at the time. 50 million of them died. Think about the 5-10 people you interacted with most recently. If this was 1918, two or three of them would be locked into a dangerous game of Russian roulette—10 chambers, 1 bullet.
Fast forward through the next 100 years… Those who were lucky enough to survive the Spanish flu plus their 7.8 billion descendants quickly encountered Asian flu, typhoid, polio, measles, HIV, malaria, dengue, cholera, swine flu, bird flu, SARS, MERS, Zika, Ebola, and now COVID-19. Not to mention countless other infectious diseases and cancer!
The question we should be asking is not how do pandemics happen, but with all the potentially fatal diseases out there, why is anybody still alive?
The question we should be asking is not how do pandemics happen, but with all the potentially fatal diseases out there, why is anybody still alive? And, more importantly, what controls who lives and who dies, and how do we shift the odds? Fortunately for your two or three hypothetical friends with the Spanish Flu, doctors started asking these questions in 1918. Without the intervention strategy they devised, the death toll might have been closer to 100 million.
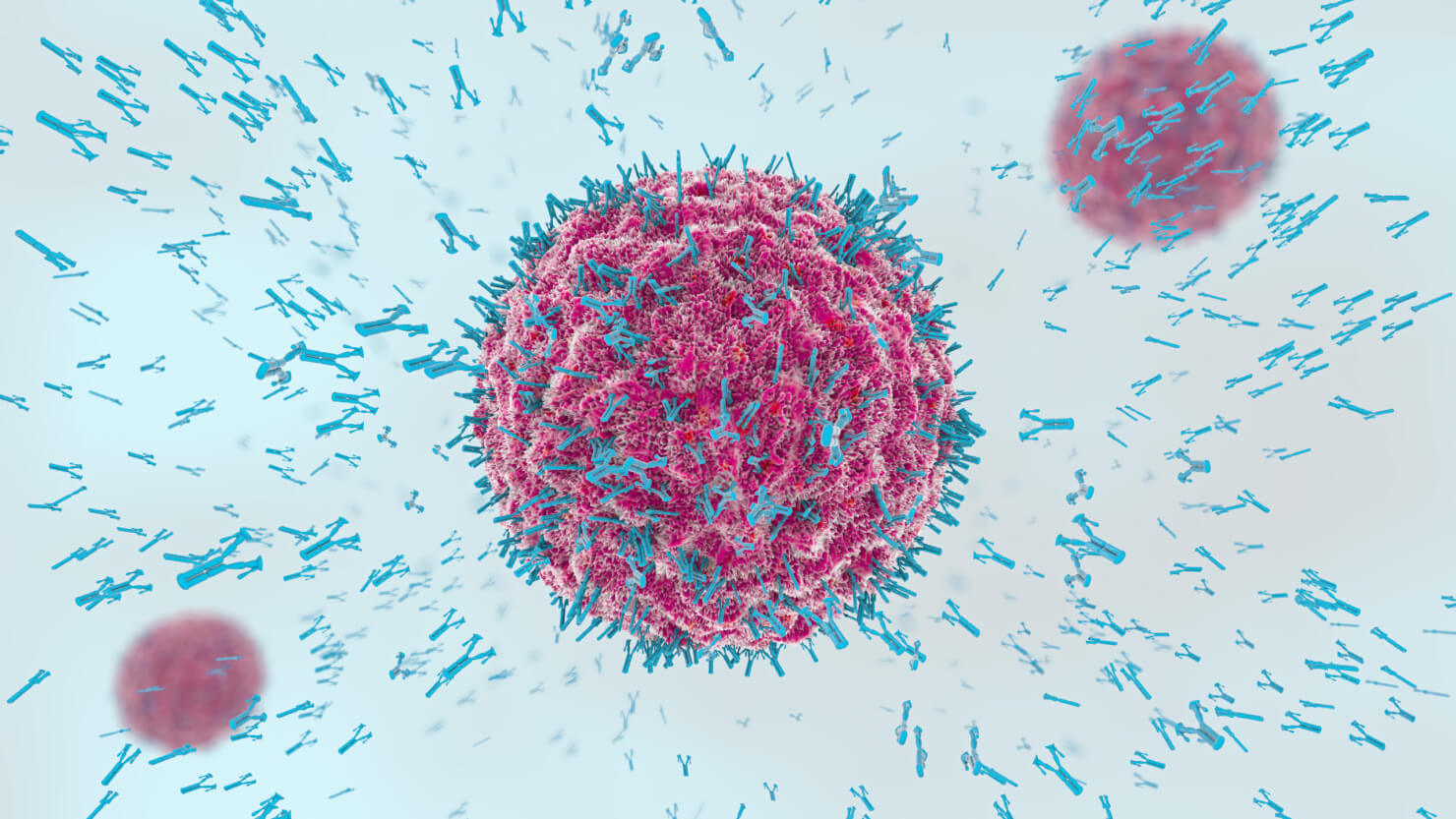
Thanks in part to pioneering work by these early pandemic doctors, we now know exactly what factor determines survival in the face of disease: tiny y-shaped proteins called antibodies that tag foreign agents for destruction. We each have the capacity to produce hundreds of thousands of antibodies.
You can think of your pool of antibodies as a massive collection of unique keys, all varying slightly in shape. The outer surface of viruses also has a unique shape that, by chance, might be complimentary to one of your antibodies. When a virus turns your body into a pathogen breeding ground, your immune system mobilizes to find a key that fits. Once a match is found, the matching key is copied over and over and over again.
So, the formula for whether or not you survive really boils down to two simple questions: 1) Do you have a matching antibody? 2) Are you young and healthy enough to make ample copies of the antibody before your system is overrun?
Now that we have a formula, the next question is how to use it to give people on the losing end of the equation a boost. Before anybody really knew what antibodies were, doctors had a hunch that whatever trait enabled some people to survive infections might be transferable. During the Spanish flu outbreak, they began injecting recently infected patients with plasma (the liquidy portion of blood that remains after cells are removed) from survivors. This practice cut fatality rates in half.
100 years later, we now know that these plasma treatments worked because of antibodies. We also know that vaccines—which give your immune system the chance to find and copy the right key before there is actually a threat—work because of antibodies. So, the obvious question is, why don’t we just give people antibodies?
Despite the quantum leaps in our understanding of immunology since 1918, one major obstacle still stands in the way of routinely producing antibody-based treatments: It’s really, really hard to find the right key when you’re searching through a pile of hundreds of thousands.
Since the time of the Spanish flu, new techniques have been developed for identifying cells that produce useful antibodies. These cells can be grown in culture, and the cells themselves or the antibodies themselves can be injected as a therapy. The resulting treatment is more potent and less prone to causing allergic reactions than straight plasma, but it’s also time consuming, expensive, and doesn’t scale well. Due to the cost, such therapies have largely been restricted to treating cancer (antibodies can also recognize cancer cells).
To better understand how the immune system mobilizes to produce antibodies amidst an infection or cancer, many immunologists are now turning to gene sequencing. Like all proteins, antibodies are encoded by DNA. By sequencing the DNA blueprint, or the functional RNA copies, of immune cells, immunologists can determine what antibodies are being produced.
Once we’ve identified the DNA sequences that encode helpful antibodies, we can produce them synthetically or via biomanufacturing (inserting the DNA into a fast-growing organism like E. coli). Producing antibodies is no easy feat—they are composed of two different building blocks that have to be produced separately and then combined without compromising shape—but considerable progress has been made on this front in the last 20 years.
Now, advanced sequencing technologies are making it easier than ever to identify the antibodies that are worth manufacturing. For instance, single cell sequencing enables researchers to identify the paired building blocks of the antibody simultaneously. Single cell immune sequencing also aids in the discovery of so-called super antibodies. Super antibodies have the ability to detect multiple related viruses (like a master key) or are exceptionally potent against cancer cells. Because they are extraordinarily rare, super antibodies are often not detected in traditional sequencing approaches that pool thousands of cells together.
Experts in the field predict that an explosion of antibody-based treatments is just over the horizon. In the short time since the first antibody-based treatment was approved in 2002 (an auto-immune drug branded as Humira), over 500 investigational drugs have been produced (79 of these were actually approved as of December 2019).
Related therapies that involve engineering cells to produce particular antibodies have also exploded. For instance, T cells, which stimulate B cells to make antibodies, can be engineered to recognize particular cancer cells in a process called CAR T-cell therapy. Since it was first described in 2010, CAR-T therapy has been used in hundreds of clinical trials.
Antibody based drugs and therapies embody what scientists and doctors have been trying to accomplish for over 100 years: using the immune system of survivors to give the immune system of struggling or vulnerable patients a boost. We are riding the first wave of what is likely to be a tsunami of antibody-based medicine. With one pandemic in our midst and likely many others at our door, it may have come just in time.